In September 2024, the Ig Nobel Prize in Physiology was awarded to Professor Takanori Takebe of the Institute of Science Tokyo (formerly Tokyo Medical and Dental University) and the research team for their study of mammals’ intestinal breathing, or enteral ventilation.
EVA Therapeutics, Inc., led by CEO Hiromu Ozaki, is developing an enteral ventilation medical device that implements this winning research in medicine.
Please tell us about EVA Therapeutics.
Professor Takebe is a young scientist in the field of regenerative medicine using induced pluripotent stem cells and is gaining international recognition. He is also quite entrepreneurial and has run many joint research projects with corporate partners.
As background, mammals such as humans normally breathe by pulmonary respiration: we inhale air through our nose and mouth, and our lungs distribute oxygen into our bloodstream. However, the research team with Prof. Takebe discovered that mammals can also take in oxygen into the systemic blood circulation through their large intestine without using their lungs, a form of respiration called enteral ventilation.
If this research finding can be established as medical technology, lives can be saved, such as patients of pulmonary diseases who suffer respiratory failure or extremely low birth weight infants who are born with underdeveloped lungs. EVA Therapeutics was established in June 2021 to apply this technology into the medical scene. Our top priority is the development of a highly anticipated neonatal medical device.
Please explain what an “enteral ventilation medical device” is.
The enteral ventilation medical device we are currently developing, the EVA-101, is similar to an IV infusion bag but with an attached enema-like nozzle. The bag is filled with the heavy liquid called perfluorodecalin (PFD) . The liquid is highly oxygenated and is infused into the patient’s intestine through the anus using a special tube to retain the fluid there.

For example, with a 3000g newborn, about 30 to 50cc of this liquid is infused each time. We believe this will enable enteral breathing to last for four to six hours. Once depleted, the liquid is discharged from the body and exchanged with a fresh dose of oxygenated liquid. If one injection works for four hours, then only six liquid exchanges are necessary per day.
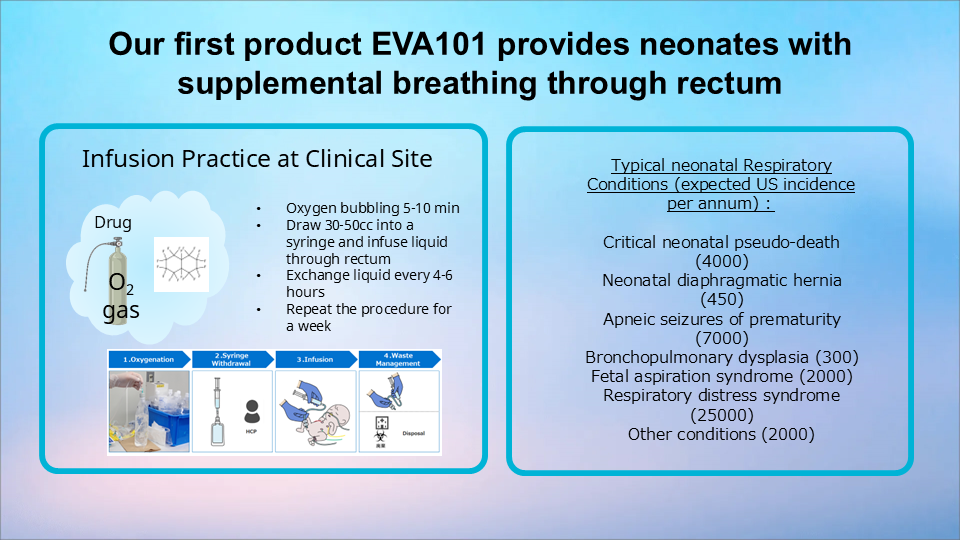
Of all babies born in Japan every year, about 15,000 newborns require respiratory assistance (*). In most cases, once these babies grow and their lungs develop, they will not require any enteral breathing measures. EVA-101 is meant to provide supplemental aid until then, and we believe more lives can be saved if we can make this technology in use. With support from professional medical organizations such as the National Center for Child Health and Development and the Institute of Science Tokyo, we will be preparing for clinical trials.
More development work will be required for an adult device because of the greater volume of liquid that needs to be exchanged. To this end, we are experimenting several new devices such as circulating the liquid continuously. A clinical trial for healthy adult males is ongoing and expected to end in March, 2025.
(* From MDV Analyzer 2023)
https://www.mdv.co.jp/solution/pharmaceutical/analyzer.html
Please take us through EVA Therapeutics’ history and where it is headed.
I worked at Fujisawa Pharmaceutical (currently Astellas Pharma Inc.) for more than 25 years on business development in North America. After that, I became the president of a few biotech ventures in the US and Japan. In 2016, I became an independent consultant specializing in pharmaceutical business development and supported Euro-American corporations entering the Japanese market.
In medicine and medical technology, copious amounts of research seeds are generated every year, but only a few can be socially implemented. Among those, this research is quite encouraging and addresses an existing social need, which is why my team and I established EVA Therapeutics.
The current challenge is development costs. On top of funding from venture capitals, we are looking into government subsidies.
Ever since Prof. Takebe and the research team received the Ig Nobel Award in 2024, enteral breathing became widely known in the general public, and our company is also being recognized. We would like to capture this momentum and step up our development so we can soon have a product.

Interview Date: October 29 (Tue.), 2024
Interviewed and written by Aya Iwamura
